Molecular Biology Notes 2023 MSc Botany 3rd Semester.The Biology Notes is an educational niche website related to biology (microbiology, biotechnology, biochemistry, zoology, botany, cell biology, genetics, molecular biology, etc.) and different other branches of biology with the aim to provide biology notes for high school, undergraduate and graduate students.
Purine is a water-soluble heterocyclic aromatic organic compound consisting of a six-membered pyrimidine ring and a five-membered imidazole ring.
A nucleotide sequence in the cell’s DNA determines the nucleotide sequence of each RNA and the amino acid sequence of each protein.
Nucleotides have three characteristic components:
- A nitrogenous (nitrogen-containing) base
- A pentose
- A phosphate

The nitrogenous bases are derivatives of two parent compounds, pyrimidine, and purine.
- Purine Structure
- Types of Purine
- Adenine
- Guanine
- Purine Derivatives
- Purine Modification
- Antibiotics and Related Compounds
- Purine Health Effects
- References
Purine Structure
- Nine atoms make up the basic purine structure.
- A six-membered pyrimidine ring and a five-membered imidazole ring fused to make up the two cycles of purine.
- Four nitrogen atoms exist in positions 1, 3, 7, and 9.
- The first nitrogen of the six-membered ring serves as the starting point for purine’s numbering, which moves counterclockwise.
- The imidazole ring is numbere in a clockwise direction.
- Purine bases are attache with 1’ carbon of pentoses through the ninth nitrogen atom to form nucleosides.
- Significant delocalization exists among the purine ring’s electrons.
- Positions 3 and 7 are electron-rich and susceptible to electrophilic attack, while positions 2, 6, and 8 are susceptible to nucleophilic attack.
Adenine
Molecular formula: C5H5N5
Molecular weight: 135.13 dalton
Melting point: 360-365°C
Solubility: 1030 mg/L (at 25 °C)
- It is a white, crystalline purine base with an amine group linked to the carbon at position 6 that is present in both RNA and DNA.
- Thymine in DNA and Uracil in RNA is complementary to adenine.
- The amine group at position 6 and the additional double bond between N-1 and C-6 in the heterocyclic aromatic (pyrimidine) ring distinguish adenine from guanine.
- The energy-rich adenosine triphosphate (ATP) and the cofactors flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide (NAD), and coenzyme A are examples of adenine derivatives that play various roles in biochemistry, including cellular respiration.
Guanine
Molecular formula: C5H5ON5
Molecular weight: 151.13 dalton
Melting point: 360°C
Solubility: 2080 mg/L (at 37 °C)
- It is a white, insoluble, crystalline material.
- It is a 2-aminopurine with a 6-oxo substituent in both RNA and DNA.
- Guanine is complementary to Cytosine in both DNA and RNA.
- It was first isolated from guano (bird manure), hence the name.
- Ammonia, a byproduct of protein breakdow in cells, is converte to guanine by spiders, scorpions, and a few other amphibians, as it can be eliminate with minimal water loss.
Purine Derivatives
Aside from adenine and guanine, other significant metabolites include hypoxanthine, xanthine, theophylline, theobromine, caffeine, uric acid, and isoguanine.
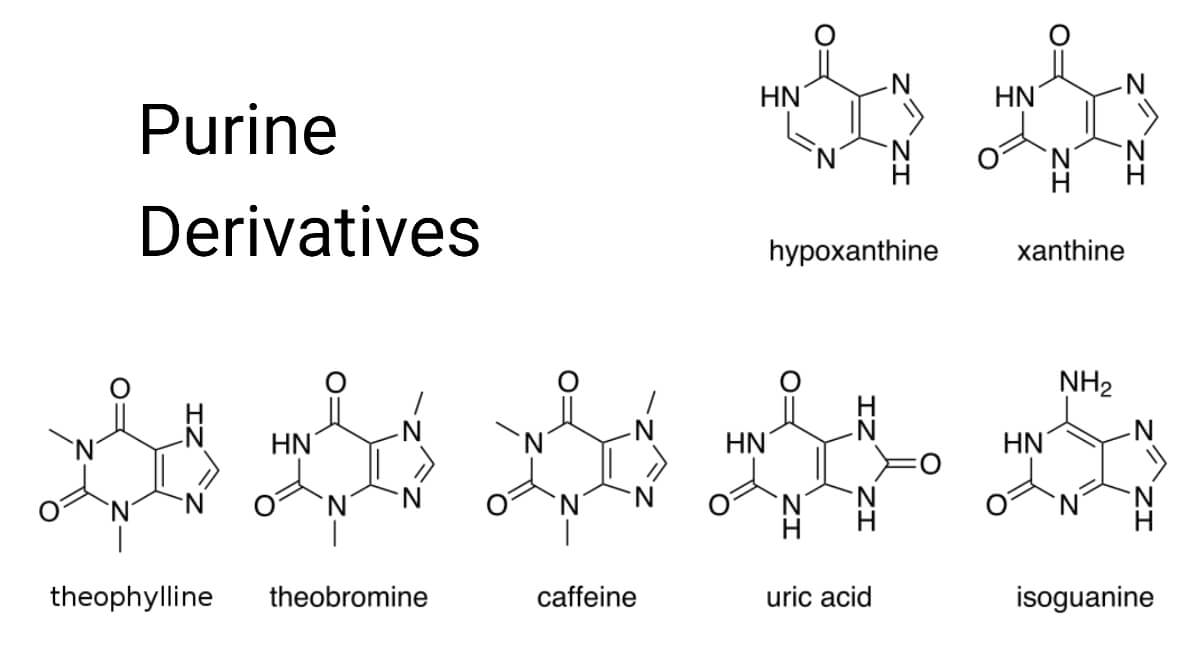
- 3, 7-dimethylxanthine (theobromine) is a powerful diuretic and facilitates oral administration by forming soluble complexes with salts of different organic acids and also in treating asthma.
- The primary purine found in coffee beans and tea leaves is 1,2,7-trimethylxanthine (caffeine) which is a cardiac and respiratory stimulant and is frequently used in headache powders.
- The main purine component of human urine is 1-methylxanthine.
- 3- and 7-Methylpurines are also minor components of urine, particularly after consuming substantial amounts of caffeine or other methylated xanthines.
- Paraxanthine, also known as 1,7-Dimethylxanthine, is an effective diuretic with antithyroid effects.
Purine Modification
- Numerous biologically active substances have been produced from modified purine structures, both of natural and synthetic origin.
- These substances often include simple alterations to known purines, such as cytokinins, 6-N-alkylated adenines, or structural changes to the carbohydrate moiety of ribose or deoxyribose derivatives, as with arabinosides.
- Methylation is the most common form of purine modification.
- Some modified purines can be found in transfer RNAs (tRNAs).
- Before the reverse shift to the B-supercoiled conformation, methylation of one of the two purines is presumably eliminated during the cell cycle.
- Methylation of purines (particularly of adenine) in DNA is known to occur in the genetic material of microorganisms. The 6-methyladenine is found in bacterial DNA.
